Plug & Produce – Lifecycle management for modular plants: Efficient data-based consistency and integrity!
The MTP4pharma® brand combines concepts, methods, tools, training and consulting services to support customers in the pharmaceutical industry and their suppliers in the legally compliant life cycle management of modular plants.
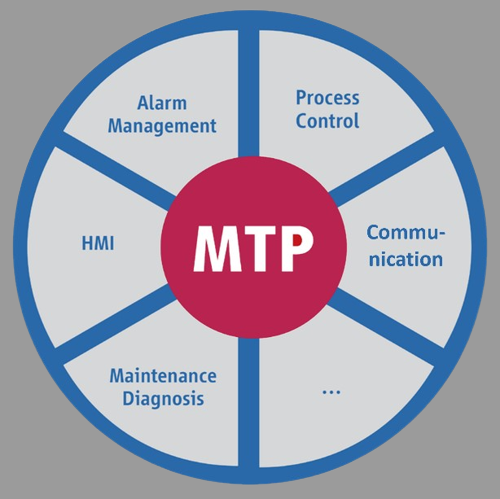
The approach is based on the concept of end-to-end dynamic engineering and semantic modelling of product-related, technical, legal and organizational requirements when mapping the life cycle of pharmaceutical plants.
To this end, semantic data models for planning, realization, qualification, validation and operation are developed together with customers, which are seamlessly based on and integrated with the MTP standard.
Semantic data models provide an abstract and formal method for describing and representing a specific reality. They aim to map elements and their relationships in a data model, whereby the level of detail can be selected flexibly.
From the outset, legal requirements are mapped as an integral part of the data model so that the degree of fulfillment of requirements can be documented at any time without significant additional effort. This is the core of the MTP4pharma® brand – intrinsic conformity, minimizing GxP compliance risks.